Characteristics of Rare Earth Elements
Rare earth elements (REE) include the 15 lanthanide elements on the periodic table from lanthanum (La) to lutetium (Lu), and also properly include the transition metals scandium (Sc) and yttrium (Y). The REE are classified as metals and are relatively soft, ductile and malleable. Most have a silver-gray luster and oxidize rapidly in air to form oxide compositions (REE)2O3. The normal trivalent ionic form and stepwise decrease in ionic radius of the lanthanides (La to Lu) and Y (Table 1) gives rise to similar geochemical properties and common associations in nature. Although Sc also shares the trivalent ionic form, the much smaller ionic size results in geochemical behavior that differs from the other REE and is more often associated with metallic elements like chromium (Cr), cobalt (Co), and nickel (Ni). The lanthanide promethium (Pm) is the only radioactive element of the series, is extremely rare in nature, and mainly produced synthetically as a byproduct of uranium fission.
The REE are commonly subdivided into light REE (LREE), which include La, Ce, Pr, Nd, Pm, Sm, Eu, and Gd, and heavy REE (HREE), including Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y (Table 1). Although a somewhat arbitrary designation, the LREE have a relatively large ionic radius similar to that of calcium (Ca2+=1.12 Angstrom) and thorium (Th4+= 1.05 Angstrom), whereas the HREE generally have a smaller ionic radius similar to uranium (U4+=1.0 Angstrom) and manganese (Mn2+=0.96 Angstrom) (Shannon, 1976). The range of ionic sizes thus allows for substitution into more than 200 rock-forming minerals, especially phosphates and carbonates. REE-bearing minerals often show selective enrichment in LREE or HREE depending upon the original composition and crystal structure of the mineral compound, the geological conditions in which the mineral formed, and a variety of complex ion exchange mechanisms.
| Rare Earth Element1 | Atomic Number | Atomic Weight | Ionic radius2 (Angstrom, CN8) | Crustal abundance3 (ppm) | Density (g/cm3) | Melting Point (F) | Hardness (Mohs) | Conductivity (W/m-1K-1) |
|---|---|---|---|---|---|---|---|---|
| Lanthanum (La) | 57 | 138.905 | 1.160 | 31 | 6.15 | 1688 | 2.5 | 13.4 |
| Cerium (Ce) | 58 | 140.116 | 1.143 | 63 | 6.77 | 1463 | 2.5 | 11.3 |
| Praseodymium (Pr) | 59 | 140.908 | 1.126 | 7.1 | 6.77 | 1715 | 1.41 | 12.5 |
| Neodymium (Nd) | 60 | 144.242 | 1.109 | 27 | 7.01 | 1875 | 1.23 | 16.5 |
| Promethium (Pm) | 61 | 145 | 1.093 | - | 7.26 | 1908 | - | 17.9 |
| Samarium (Sm) | 62 | 150.36 | 1.079 | 4.7 | 7.52 | 1962 | 1.44 | 13.3 |
| Europium (Eu) | 63 | 151.964 | 1.066 | 1.0 | 5.24 | 1519 | 3.07 | 13.9 |
| Gadolinium (Gd) | 64 | 157.25 | 1.053 | 4.0 | 7.90 | 2394 | 5.13 | 10.6 |
| Terbium (Tb) | 65 | 158.925 | 1.040 | 0.7 | 8.23 | 2473 | 2.33 | 11.1 |
| Dysprosium (Dy) | 66 | 162.500 | 1.027 | 3.9 | 8.55 | 2565 | 1.8 | 10.7 |
| Holmium (Ho) | 67 | 164.930 | 1.015 | 0.83 | 8.79 | 2662 | 1.65 | 16.2 |
| Erbium (Er) | 68 | 167.259 | 1.004 | 2.3 | 9.07 | 2784 | 1.97 | 14.5 |
| Thulium (Tm) | 69 | 168.934 | 0.994 | 0.30 | 9.32 | 2813 | 1.77 | 16.9 |
| Ytterbium (Yb) | 70 | 173.045 | 0.985 | 1.96 | 6.90 | 1515 | N/A | 38.5 |
| Lutetium (Lu) | 71 | 174.967 | 0.977 | 0.31 | 9.84 | 3006 | 2.6 | 16.4 | Scandium (Sc) | 21 | 44.956 | 0.870 | 14.0 | 2.99 | 2806 | N/A | 15.8 |
| Yttrium (Y) | 39 | 88.906 | 1.019 | 21 | 4.47 | 2779 | N/A | 17.2 |
Table 1: Basic properties of rare earth elements (REE)
- LREE commonly include La to Gd, HREE include Tb to Lu, Sc, Y.
- Trivalent ionic radius in Angstroms (Shannon, 1976); CN8 = coordination number VIII.
- Rudnick and Gao, 2003
Of the more than 200 minerals containing REE in trace quantities or greater, only a few contain enrichments that have the potential for forming economic ore deposits. The important REE ore minerals are listed in Table 2 with the commonly accepted chemical formula and theoretical REE-oxide content. The specific REE composition can vary. Occurrences of all of these minerals have been noted in Virginia.
| Mineral Name | Chemical Formula | Specific Gravity g/cm3 | 1Theoretical (REE)2O3 % |
|---|---|---|---|
| Bastnaesite | (Ce,La)(CO3)F | 4.98 | 74.81 |
| Monazite | (Ce,La,Nd,Th)PO4 | 5.04-5.15 | 69.73 |
| Xenotime | YPO4 | 3.14-4.27 | 61.40 |
| Parisite | Ca(Ce,La)2(CO3)3F2 | 4.34 | 60.89 |
| Ancylite | CeSr(CO3)2(OH)▪H2O | 3.95 | 47.98 |
| Florencite | CeAl3(PO4)2(OH)6 | 3.58 | 31.99 |
| Euxenite | (Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6 | 5.3-5.9 | 25.77 |
| Fergusonite | (Nd,Ce)(Nb,Ti)O4 | 4.5-5.7 | 18.86 |
| Apatite | Ca5(PO4)3(OH)(F,Cl) | 3.16-3.22 | variable up to ~19 |
| Allanite | (Ce,Ca,Y)2(Al,Fe3+)3(SiO4)3(OH) | 3.5-4.2 | variable up to ~17 |
Table 2: Economic minerals containing rare earth elements
- Mariano, 1989.

The REE neodymium is used for
clean energy technology
Uses of Rare Earth Elements
In 1787 an amateur geologist found a strange looking mineral in a Swedish quarry. The mineral, gadolinite, contained the first identified rare earth element, yttrium. Over the next several decades, the other REE were discovered. Today, REE are considered "critical minerals" in domestic metallurgical applications that serve aerospace, defense, energy, telecommunications, electronics, and transportation technologies (Fortier and others, 2018) many sectors of the economy benefit from REE technology. REE are used as catalysts in chemical reactions (especially for the production of petroleum), in the production of glass and ceramics, and in fiber optics. Clean energy technologies frequently require various REE, such as neodymium for electric car engines and wind turbine generators. Dysprosium, gadolinium, praseodymium, samarium, and neodymium are used to produce strong magnets for computers and cell phones. Cerium, neodymium, samarium, and praseodymium are also important for rechargeable batteries. The medical industry requires erbium, gadolinium, thulium, ytterbium, and yttrium for x-ray and MRI equipment, laser surgery, and cancer treatment. Cerium, lanthanum, and neodymium are important alloys.
| Rare Earth Element | Characteristics | Use |
|---|---|---|
| Cerium (Ce) | Soft, ductile | Alloys, polishing, colorant, catalyst, lighters, turbine blade coating |
| Dysprosium (Dy) | Soft, magnetic properties | Lasers, nuclear reactors, magnets, computers |
| Erbium (Er) | Soft, electropositive | Laser surgery, fiber optics |
| Europium (Eu) | Soft, highly reactive, rare | TV screens, nuclear reactors, lasers, fluorescent lamps |
| Gadolinium (Gd) | Ductile, magnetic properties | Neutron shielding, magnetic resonance imaging (MRI), lasers, computers |
| Holmium (Ho) | Soft, high magnetic strength | Magnetic fields, lasers, colorant |
| Lanthanum (La) | Soft, highly reactive | Catalysts, lighters, lamps, optics, alloys |
| Lutetium (Lu) | Reactive | Catalysts, positron emission tomography (PET) |
| Neodymium (Nd) | Reactive, hard, malleable | Lasers, magnets, computers, electric motors, generators, lighting, alloys |
| Praseodymium (Pr) | Soft, ductile, malleable,magnetic | Magnets, colorant, lasers, flints |
| Promethium (Pm) | Radioactive, rare, reactive | Paints, nuclear batteries |
| Samarium (Sm) | Hard, reactive, magnetic | Magnets, lasers, nuclear reactor control rods |
| Scandium (Sc) | Soft, reactive | Aerospace alloys, light bulbs |
| Terbium (Tb) | Soft, malleable, ductile,magnetic | Magnets, lasers, fluorescent lamps, alloys, sonar technology, fuel cells |
| Thulium (Tm) | Radioactive, soft, malleable | X-ray technology, lasers |
| Ytterbium (Yb) | Soft, malleable, ductile | Lasers, nuclear medicine, seismology, alloys |
| Yttrium (Y) | Soft, reactive | Lasers, TVs, superconductors, LEDs, light bulbs, cancer treatment |
Table 3: Characteristics and uses of rare earth elements
Rare earth elements Geology

Synchysite crystals.
Photo credit: Christian Rewitzer
https://commons.wikimedia.org/wiki/File:Synchysite-107154.jpg
Despite the group name, most of the rare earth elements are relatively abundant in the Earth's crustal rocks, although rarely as native metals. For comparison, the average crustal concentration of lanthanum is 31 parts per million (ppm) (Table 1), slightly greater than the average for copper (Cu), which is 28 ppm (Rudnick and Gao, 2003). The least abundant naturally-occurring REE, thulium, has an average concentration of 0.30 ppm, which is still substantially higher than the silver content in the Earth's crust, which is 0.053 ppm.
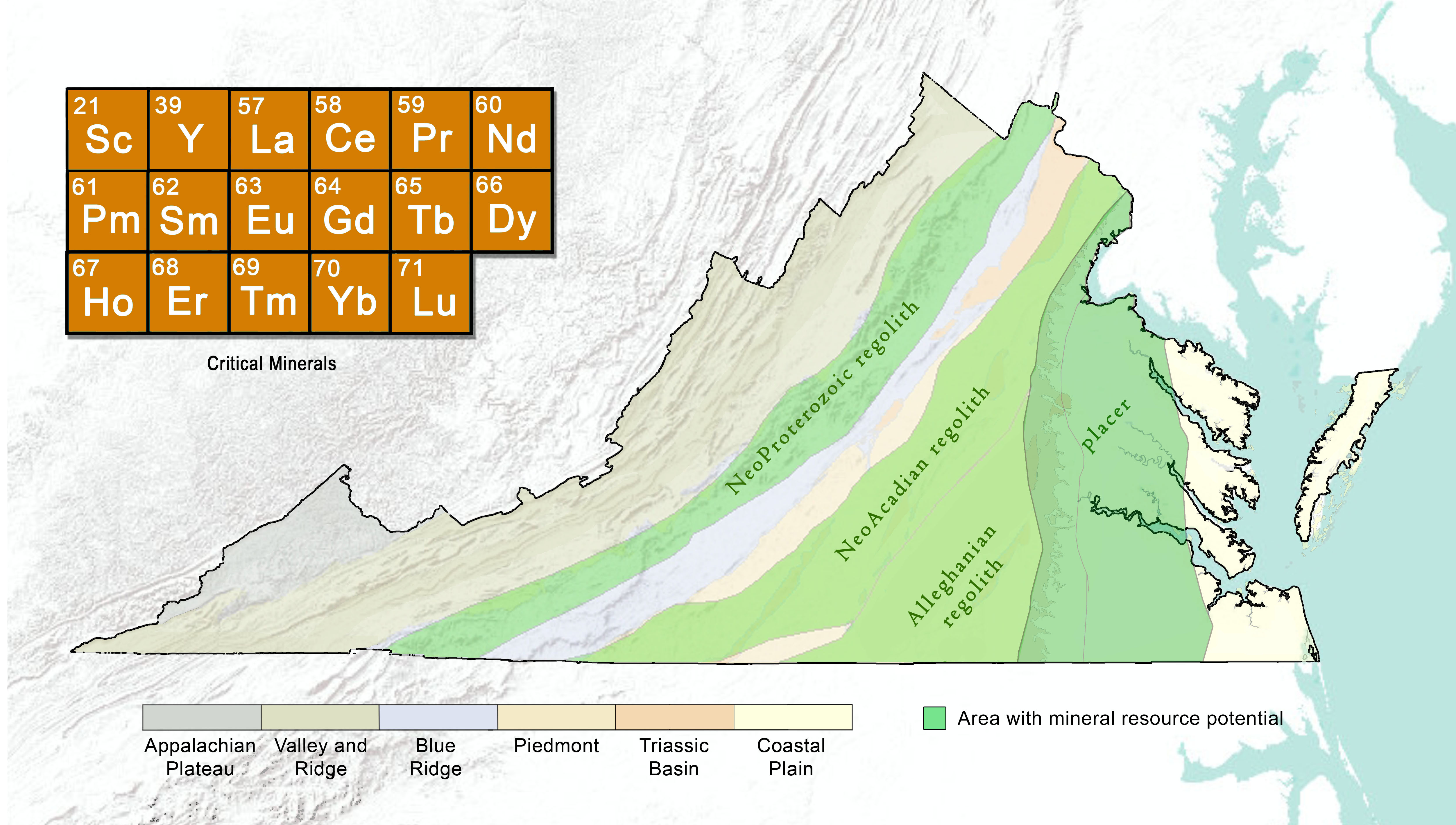
The relatively large ionic size and charge balance characteristics of the REE in magmatic systems generally preclude their inclusion in most of the common rock forming minerals including feldspars, quartz, amphiboles, mica, etc. Consequently, the REE tend to concentrate in rocks formed by magmas that have undergone a high degree of fractionation. These rocks include alkaline and silicic igneous complexes, pegmatites, felsic volcanics, and carbonatites. Because of the close similarity in ionic sizes, thorium and uranium are frequently associated with REE. High temperature hydrothermal fluids, especially those enriched in chlorine, fluorine and lithium can also mobilize REE. Economic deposits of REE are found in magmatic systems, magmatic magnetite-hematite or iron oxide-copper-gold (IOCG) deposits, in heavy mineral placers, and in chemical weathering zones within ion-adsorption clay deposits (USGS, 2020).
Virginia Energy's Geology and Mineral Resources Program is currently conducting a study to evaluate rare earth element enrichment and mobilization in regolith above granitoid plutons across the Piedmont. This study which is led by the North Carolina Geological Survey and partnering with the South Carolina Geological Survey is one of the first of its kind to assess the regional REE geochemistry in the southeastern United States in the context of ion-clay adsorption deposits. More details are available on our project webpage (Earth MRI).
| Mineral System | Deposit Type | Geologic Provinces |
|---|---|---|
| Placer | heavy mineral sands | Coastal Plain, Outer Continental Shelf |
| Placer | Paleo-placer | Blue Ridge, Piedmont |
| Marine chemocline | Phosphate | Coastal Plain |
| Magmatic REE | magmatic Ti-Fe-P (anorthosite, nelsonite) | Blue Ridge, Piedmont |
| Chemical weathering | regolith (ion adsorption) concentration in saprolite | Blue Ridge, Piedmont |
Table 4: Prospective REE mineral systems, deposit types (Hofstra and Kreiner, 2020), and geologic provinces in Virginia

Xenotime crystals.
Photo credit: Robert Lavinsky
Rare Earth Elements in Industry
Although the U.S. maintains a supply of REE in the National Defense Stockpile, the nation is presently 100 percent reliant on imports, mainly from China, Estonia, Japan, and Malaysia. The Mountain Pass mine in California is currently the only domestic producer of REE concentrates, yet all of this material is shipped to China for processing, refining, and manufacturing of batteries, magnets and other high-tech products. Geologic resources have been identified in Alaska, Arizona, Idaho, Montana, Missouri, Nebraska, Nevada, Texas, and Wyoming (USGS, 2020). Concerns related to mining rare earths include the possible contamination of water and soil near processing sites (USGS, 2020).
In Virginia, REE can be found in relatively low concentrations in many different geologic rock types. Source rocks for REE include bedrock granitic intrusives and granitic gneisses (common in the Piedmont, for example). Sedimentary deposits are a more likely source for REE because they are more accessible and more concentrated than the REE within metamorphic and igneous rocks.
Sedimentary sources of REE include modern and paleo-placer deposits that occur in the Atlantic Coastal Plain from Virginia south through the Carolinas and into Georgia (Mertie, 1975). These placer deposits contain heavy mineral sands such as ilmenite, rutile, and zircon, and also the REE-enriched minerals monazite and xenotime. Monazite, in particular was identified in 1849 as an important source for heavy minerals in Virginia (Mertie, 1975). Economic deposits of heavy mineral sands, including REE-enriched minerals, have been mined in Virginia in the past, and the potential for new discoveries is considered very high.

Bastnasite crystals.
Photo credit: Robert Lavinsky http://mindat.org/photo-177535.html
Although hard rock REE occurrences are known in Virginia, the extent of these deposits is limited and the concentrations are not currently economically viable. Only one site has been prospected specifically for monazite (Chestnut Knob), and all other locations described below were mined for other commodities, such as titanium and zirconium, but may also contain minor amounts of REEs.
It is possible that REE may also be concentrated in the regolith that forms from chemical weathering of REE-enriched igneous rocks. The clay deposits that result from the breakdown of bedrock minerals may retain REE by processes such as ion-adsorption. When REE containing granitic host rocks are intensely weathered, a thick clay regolith forms above the deteriorating hard rock. The rare earth elements may become concentrated within the clay regolith.
Some of the significant occurrences of REE in Virginia are described in the following sections. Although no commercial production of REE has occurred at these sites, the potential for new discoveries will be evaluated in the upcoming year.
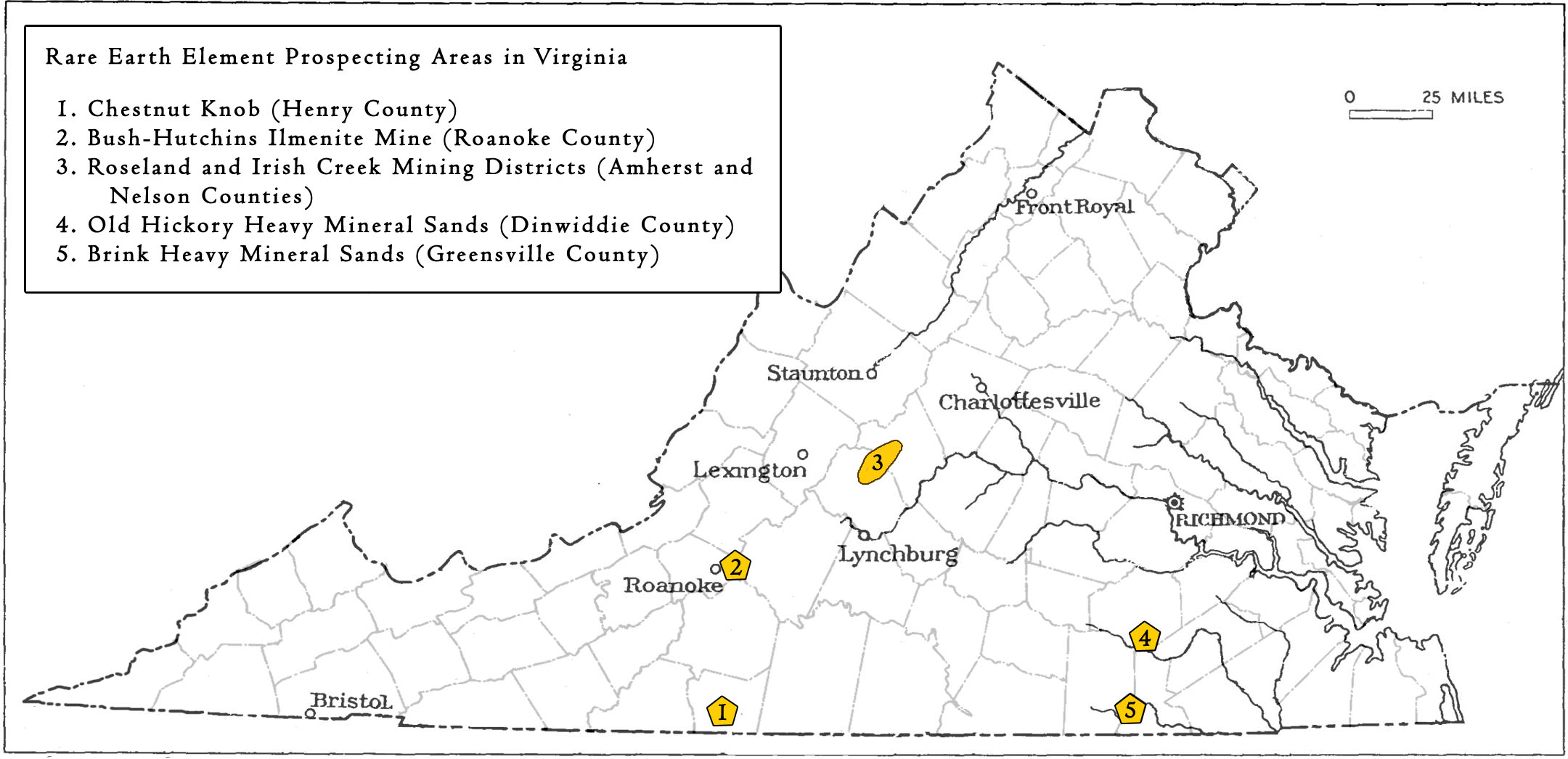
Locations in Virginia mined or prospected for REE
Dinwiddie and Greensville Counties
In Dinwiddie and Greensville counties, economic heavy mineral sands occur as paleoplacer deposits along a now exposed ancient coastline. The heavy minerals were naturally concentrated in Pliocene-age shoreline beach and dune sands by wind and wave action. The key heavy minerals in these deposits include ilmenite, rutile, zircon, monazite, and leucoxene. In 1997, RGC Resources (now Iluka Resources Ltd) began mining and processing the heavy mineral sands from the Old Hickory deposit, located in Dinwiddie County. A second mine (Brink) was permitted about 19 miles to the south in Greensville County in 2008. At both sites, heavy mineral sands were mined by excavator and then processed through gravity spiral concentrators to produce mineral concentrates. Mining operations ceased in 2017 and the sites are now undergoing reclamation. Over 6.2 million tons of heavy mineral concentrates were produced from 1997 to 2017. In addition to the key heavy minerals, these sites may be considered a potential source for REE-enriched minerals.
As of 2024, Atlantic Strategic Minerals have restarted mining operations at their Millrun Mine near Stony Creek, VA (https://www.atlanticstrategicminerals.com/). Additional investigations regarding heavy mineral sands hosting potential REE resources have been completed as part of the Earth MRI program.
Henry CountyNear the town of Ridgeway in Henry County, a deposit of monazite was discovered in 1950. By 1953, rare earth metals were positively identified and a plan to mine the Chestnut Knob prospect was established by Southern Minerals Company (Martinsville Bulletin, 1957). In 1955, the mineral rights were leased to Virginia Minerals Inc., which had become interested in the Chestnut Knob area due to promising radiometric survey results. By 1957, a 10-ft deep and 30-ft long trench was dug within which monazite-containing veins were identified (Gooch, 1958). Approximately 15 tons of monazite concentrates were removed for analysis and processing (Gooch, 1958). Between 1957 and 1960 monazite, magnetite, and zircon were mined. The ore contained 4-5 percent monazite. A pilot plant was constructed to refine the monazite. Approximately 275 tons of concentrate were shipped in from other locations to be processed at the plant, with a reported output of 100 tons of ore per day, yielding 4.5 tons of monazite with a 5 percent thorium content. Virginia Minerals Inc., which owned approximately 3,000 acres in the Chestnut Knob area, estimated up to 1,700 tons of possible monazite reserves based on exploratory drilling (Martinsville Bulletin, 1957). Although prospecting seemed promising, operations were short lived, the area was never developed and extraction ceased during the early 1960s.
The Chestnut Knob ore deposit is a placer in Neoproterozoic gneiss and schist mapped as the Fork Mountain Formation. Veins or stringers of monazite ore were noted throughout the prospecting area. Chemical analysis of the ore indicated 69 percent magnetite, 15 percent ilmenite, 9 percent monazite, and 3 precent zircon (Mertie 1955).
Roanoke CountyIn Roanoke County, occurrence of heavy minerals were prospected at the Bush-Hutchins mine. The bedrock is primarily greenstone with syenite and intrusions of nelsonite dikes. The rock nelsonite containing ilmenite and apatite was first identified in this area as early as 1890, when it was of interest for iron mining (Hickman, 1947). At that time, a few thousand tons of iron were mined and processed at local furnaces (Hickman, 1947). The mine operated on a small scale and was used intermittently with a total production of 200,000 tons of ore (Hickman, 1947). Primarily sourced from a large open cut (500 ft long, 75 ft wide, and 60 ft deep), ore was also extracted from four smaller open cuts and several prospecting pits (Hickman, 1947). Titanium in the mineral ilmenite was the primary commodity and some ilmenite-rich ore samples were mined and shipped to Richmond for processing (Watson and Taber, 1913). Although there are records of subsequent mineral prospecting in the area, the site was reported by Watson and Taber (1913) to have been abandoned during the early 20th century. Eight carloads of ore, however, were reportedly extracted in 1943 (Hickman, 1947). The site was revisited when four exploratory holes were drilled by the U.S. Bureau of Mines in 1946 following a promising geophysical survey (Hickman, 1947). Cuttings from the holes revealed up to 19.1 percent TiO2 in stringers of nelsonite. The presence of apatite in the ores at this site may indicate REE content that has not been evaluated.
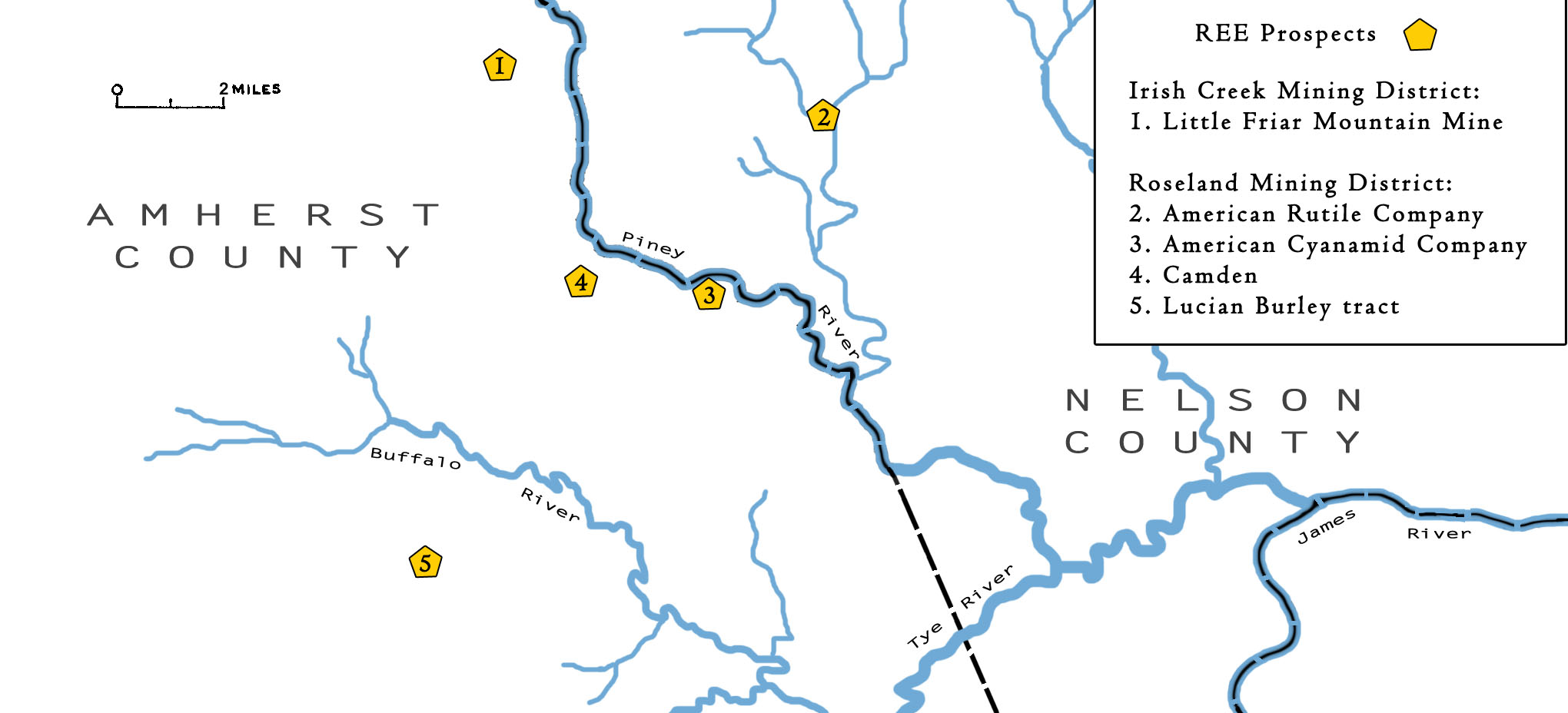
In the Blue Ridge province, several historic mine sites containing REE-enriched minerals exist in Amherst County. Although these sites were not mined specifically for REE, they may exist as a secondary commodity.
Little Friar Mountain Mine (also known as Massie Mine) near the town of Massies Mill in Amherst County is part of the Irish Creek Tin Mining District, which was discovered for its mineral resources prior to 1900. The linear ore body is approximately 150 ft long and 20 ft wide, and contains allanite, microlite, and fergusonite, which were mined for REE-oxides, as well as feldspar, niobium, and tantalum during the late 19th century and in 1925 (Mallet, 1877). The ore body is a pegmatite dike in hypersthene granodiorite. Allanite and fergusonite are sourced primarily from the central portion of the dike which is approximately 2-6 ft wide. Analysis reveals 6.5 precent cerium oxides and 6.42 percent yttrium oxides within allanite samples, and 27.94 percent erbium-yttrium oxides, 1.37 percent cerium oxides, 3.92 percent lanthanum oxides, and 4.06 percent dysprosium oxides within Fergusonite samples.
The Piney River-Roseland Mining District encompasses several mines in Amherst and Nelson counties, including the American Rutile Company mine, the American Cyanamid Company mines, Camden mine, and the Lucian Burley tract. These sites were prospected or mined mostly during the first half of the 20th century. Numerous pits and trenches were dug mainly in saprolite with nelsonite dikes containing ilmenite, allanite, rutile, helvite, and fergusonite. Although several of these sites produced ore, few records exist. (Pegau, 1932; Fish and Swanson, 1958).
Selected References:
Berquist, C.R. Jr., 1990, Chemical analyses of offshore heavy-mineral samples, Virginia inner continental shelf. In: Berquist, C.R., Jr., (ed), Heavy mineral studies - Virginia inner continental shelf: Virginia Division of Mineral Resources Publication 103, p. 109- 124.
Conley, J.F., and Toewe, E.C., 1968, Geology of the Martinsville West quadrangle, Virginia: Virginia Division of Mineral Resources Report of Investigations 16, 44 p.
Conley, J.F., and Henika, W.S., 1973, Geology of the Snow Creek, Martinsville East, Price, and Spray quadrangles, Virginia: Virginia Division of Mineral Resources Report of Investigations 33, 71 p.
Dietrich, R.V., 1990, Minerals of Virginia, 1990: Department of Mines, Minerals and Energy Division of Mineral Resources, Charlottesville, VA, 474 p.
Dietrich, R. V., 1955, Additions to Virginia mineral localities: Virginia Poly. Inst., Eng. Expt. Sta. Ser. no. 105, 31 p.
Fortier, S.M., Nassar, N.T., Lederer, G.W., Brainard, J., Gambogi, J., and McCullough, E.A., 2018, Draft Critical Mineral List - Summary of Methodology and Background Information - U.S. Geological Survey Technical Input Document in Response to Secretarial Order No. 3359: U.S. Geological Survey Open-File Report 2018-1021, 15 p.
Hawkins, D.W. and Lassetter, W.L., 2022, Geologic map compilation and geochemical investigations in the Earth MRI Virginia Fall Zone placer focus area. Virginia Department of Energy, Geology and Mineral Resources Program Open-file Report 2022-01, 50 p. and Appendices.
Gooch, E.O., 1958, Field Investigation Memorandum - Chestnut Knob monazite occurrences, Henry County, VA: Virginia Division of Mineral Resources, Economic Geology Files (unpublished), 1 p.
Hickman, R.C., 1947, Bush-Hutchins ilmenite, Roanoke County, Virginia: U.S. Bureau of Mines Report of Investigations 4112, 5 p.
Hofstra, A.H., and Kreiner, D.C., 2020, Systems-Deposits-Commodities-Critical Minerals Table for the Earth Mapping Resources Initiative: U.S. Geological Survey Open-File Report 2020-1042.
Pegau, A. A., 1932, Pegmatite Deposits of Virginia, Virginia Geological Survey Bulletin 33.
Shah A.K., Bern, C.R., Van Gosen, B.S., Daniels, D.L., Benzel, W.M., Budahn, J.R., Ellefsen, K.J., Karst, A., and Davis, R., 2017, Rare earth mineral potential in the southeastern U.S. Coastal Plain from integrated geophysical, geochemical, and geological approaches. The Geological Society of America Bulletin.
Mariano, A. N., 1989, Economic geology of rare earth elements, Chapter 11, in Lipin, B.R., and McKay, G.A., eds., Geochemistry and Mineralogy of Rare Earth Elements, Reviews in Mineralogy, Volume 21: The Mineralogical Society of America, Washington, DC, p. 309-348.
Martinsville Bulletin (unnamed writer), 1957, Large rare metal deposit located at Chestnut Knob: Martinsville Bulletin, Sunday, December 15, 1957, p. 1A, 5A.
Mertie, J.B., Jr., 1953, Monazite deposits of the southeastern Atlantic states: U.S. Geological Survey Circular 237, 31 p.
Mertie, J.B., Jr., 1955, Ancient monazite placer [abs]: Geological Society of America Bulletin, v. 66, p. 1692.
Mertie, J.B., Jr., 1975, Monazite placers in the Southeastern Atlantic States. Geological Survey Bulletin 1390.
Mertie, J.B., Jr., 1979, Monazite in the granitic rocks of the southeastern Atlantic states - an example of the use of heavy minerals in geologic exploration: U.S. Geological Survey Professional Paper 1094, 79 p.Rudnick, R.L., and Gao, S., 2003, Composition of the continental crust, in Holland, H.D., and Turekian, K.K., eds., Treatise on geochemistry, Vol 3: Elsevier Pergamon, Oxford, p. 1-64.
Shannon, R.D., 1976, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides: Acta Cryst. A32 p. 751-767.
Van Gosen, Bradley S., Philip L. Verplanck, Robert R. Seal II, Keith R. Long, and Joseph Gambogi, 2017, Rare-Earth Elements, Chapter O of Critical Mineral Resources of the United States - Economic and Environmental Geology and Prospects for Future Supply. USGS professional Paper 1802-O.
Van Gosen, Bradley S., Philip L. Verplanck, and Paul Emsbo, 2019, Rare Earth Element Mineral Deposits in the United States. USGS Circular 1454.
Van Gosen, B.S., Verplanck, P.L., Seal, R.R., II, Long, K.R., and Gambogi, J., 2017. U. S. Geological Survey Professional Paper 1802-O.
.jpg)
