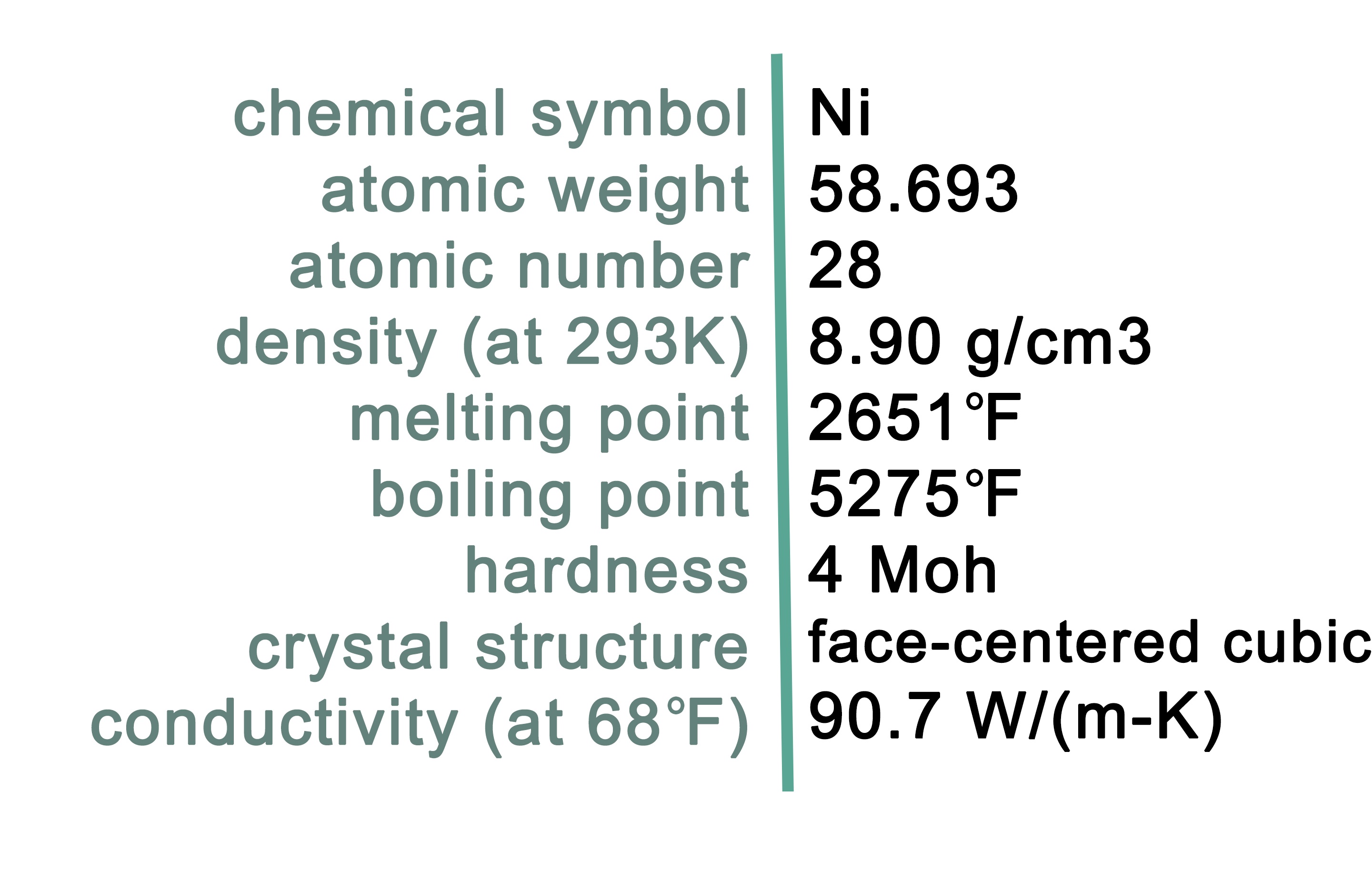
Characteristics of Nickel
Nickel is a silver-white metal with high ductility, toughness, and strength with the chemical symbol Ni. It is the 5th most abundant element and occurs extensively in the Earth's crust and core. Pure nickel is found in the Earth's crust in trace amounts, usually associated with ultramafic rock types. The Earth's core is believed to be composed primarily of an alloy of iron and nickel. Nickel also occurs in small quantities in plants, animals, and seawater. Nickel-enriched minerals that typically occur in economic deposits include a variety of sulfides that are listed in Table 1.

| Mineral Name | Chemical Formula | Specific Gravity | Ni % |
|---|---|---|---|
| Millerite | NiS | 5.3-5.5 gm/cc | 64.7 |
| Pentlandite | (Fe,Ni)9S8 | 4.6-5.0 gm/cc | 34.2; up to 67.3 |
| Pyrrhotite | (Fe,Ni)1-xSx | 4.6 - 4.65 gm/cc | trace |
| Garnierite | (NiMg)3Si2O5(OH)4 | 2.5-3.0 gm/cc | 38.9 |
| Nickeliferous limonite | (Fe,Ni)O(OH) | 2.7-4.3 gm/cc | 64.0 |
| Nickeliferous goethite | (Fe,Ni)O(OH) | 2.5-3.0 gm/cc | 64.0 |
| Violarite | FeNi2S4 | 4.0 gm/cc | 38.9 |
| Siegenite | (Ni,Co)3S4 | 4.85 gm/cc | 57.8 |
| Nickeline | NiAs | 7.5 gm/cc | 43.9 |
| Siegenite | NiS2 | 4.45 gm/cc | 47.8 |
Table 1: Minerals containing the element Nickel

High-power Ni-MH battery from Toyota hybrid vehicles
Uses of Nickel
Nickel is considered a "critical mineral" because of its high demand for wind and solar power and electric vehicles (EV). It is used in rechargeable nickel-cadmium batteries and nickel-metal hydride batteries in hybrid vehicles. As a good thermal conductor, nickel is used in making alloys to resist corrosion. For example, nichrome that is used in toasters and electric ovens is an alloy of nickel and chromium with small amounts of silicon, manganese, and iron. A copper-nickel alloy is commonly used in desalination plants, which converts seawater into fresh water. Nickel steel is used for armour plating. Other alloys of nickel are used in boat propeller shafts and turbine blades. Nickel has a long history of being used in coins. Finely divided nickel is used as a catalyst for hydrogenating vegetable oils.

The rock Garnierite, a green nickel ore found in weathered and serpentinized ultramafic rocks
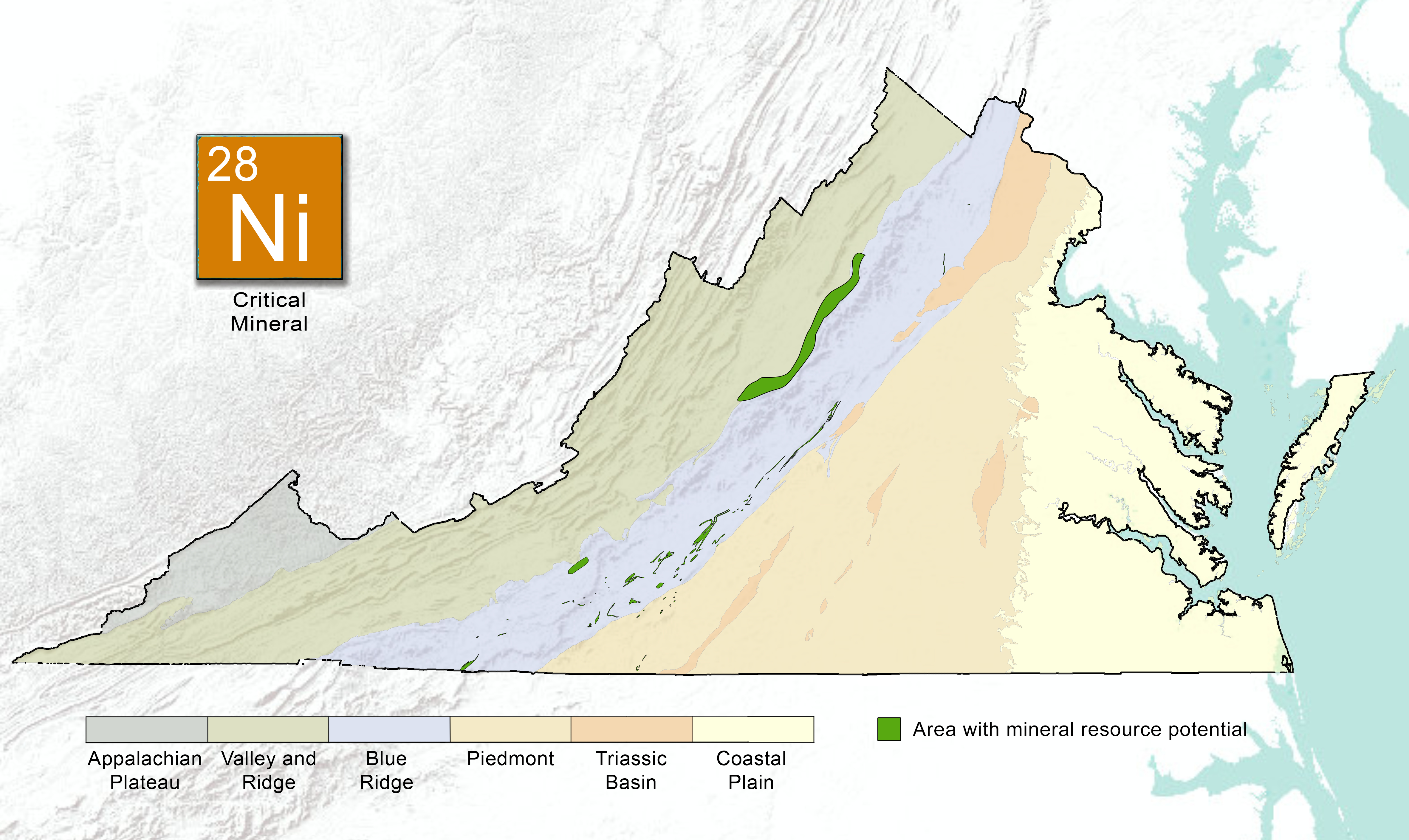
Nickel Geology
In the Earth's crust, nickel abundance averages 80 parts per million (ppm), however, it is greatly concentrated in the core. Nickel may substitute for other elements such as iron and cobalt in many minerals. On Earth, nickel occurs most often in combination with sulfur and iron in pentlandite, with sulfur in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur in nickel galena (Table 1).
| Mineral System | Deposit Type | Geologic Provinces |
|---|---|---|
| Chemical weathering | Residual nickel with clays, replacement, and supergene enrichment | Valley and Ridge, Piedmont |
| Mafic Magmatic | Sulfide | Blue Ridge |
Table 2: Prospective nickel mineral systems, deposit types (Hofstra and Kreiner, 2020), and geologic provinces in Virginia
Nickel in Industry
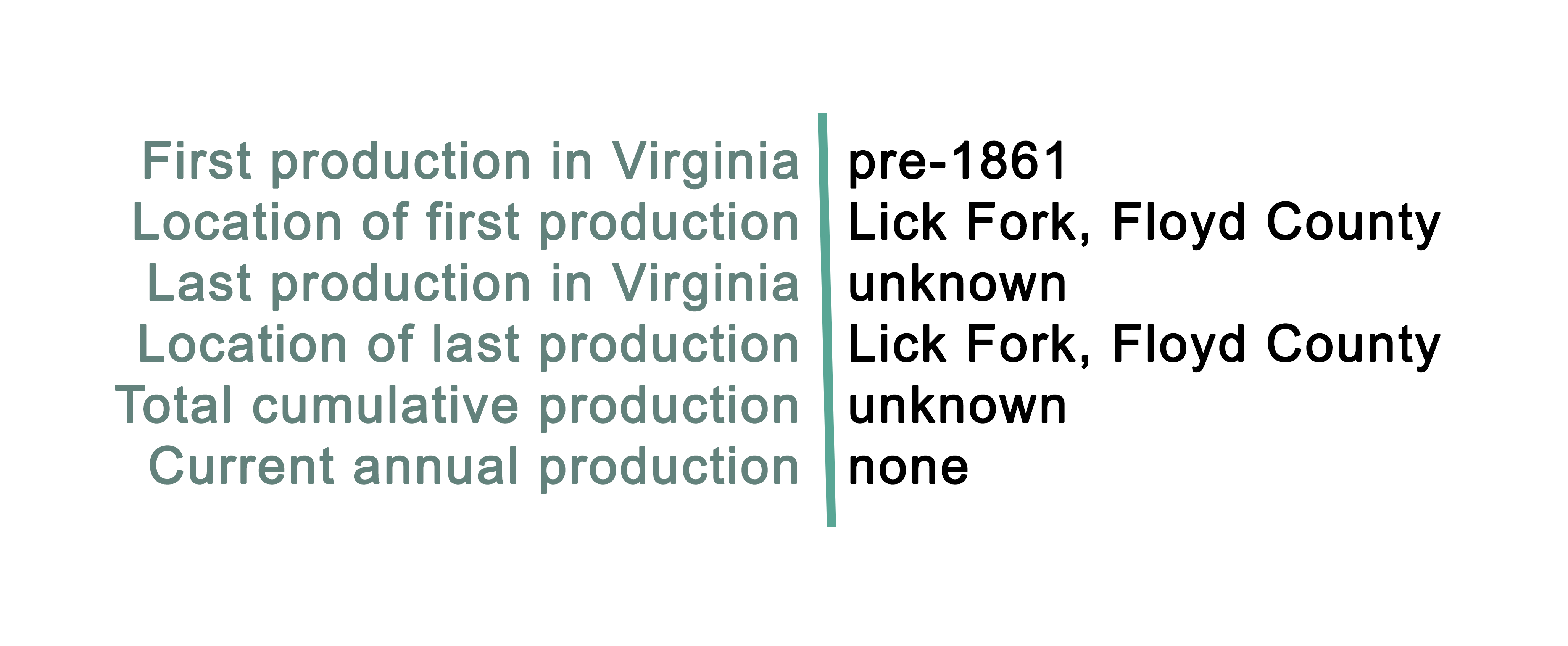
Nickel is mined from two main types of ore deposits. The first is laterite, where the principal ore mineral mixtures are nickeliferous limonite and garnierite (a mixture of various hydrous nickel and nickel-rich silicates). The second is magmatic sulfide deposits, where the principal ore mineral is pentlandite. Indonesia and Australia have the largest estimated reserves of nickel (43.6% of the world total). The U.S. currently has one domestic nickel mine, the Eagle Mine, an underground mine located in Michigan. In Virginia, nickel occurrences located in northern Floyd County were prospected before the Civil War, and again in the early years of the twentieth century. The mineralized rock includes gabbro dikes containing nickel-bearing pyrrhotite and chalcopyrite. These sulfide ores are found in veins, or irregularly distributed throughout the metamorphosed volcanic or magmatic rocks in the Blue Ridge Province. Nickel is often associated with other sulfide or metallic elements such as cobalt, iron, copper, and arsenic.
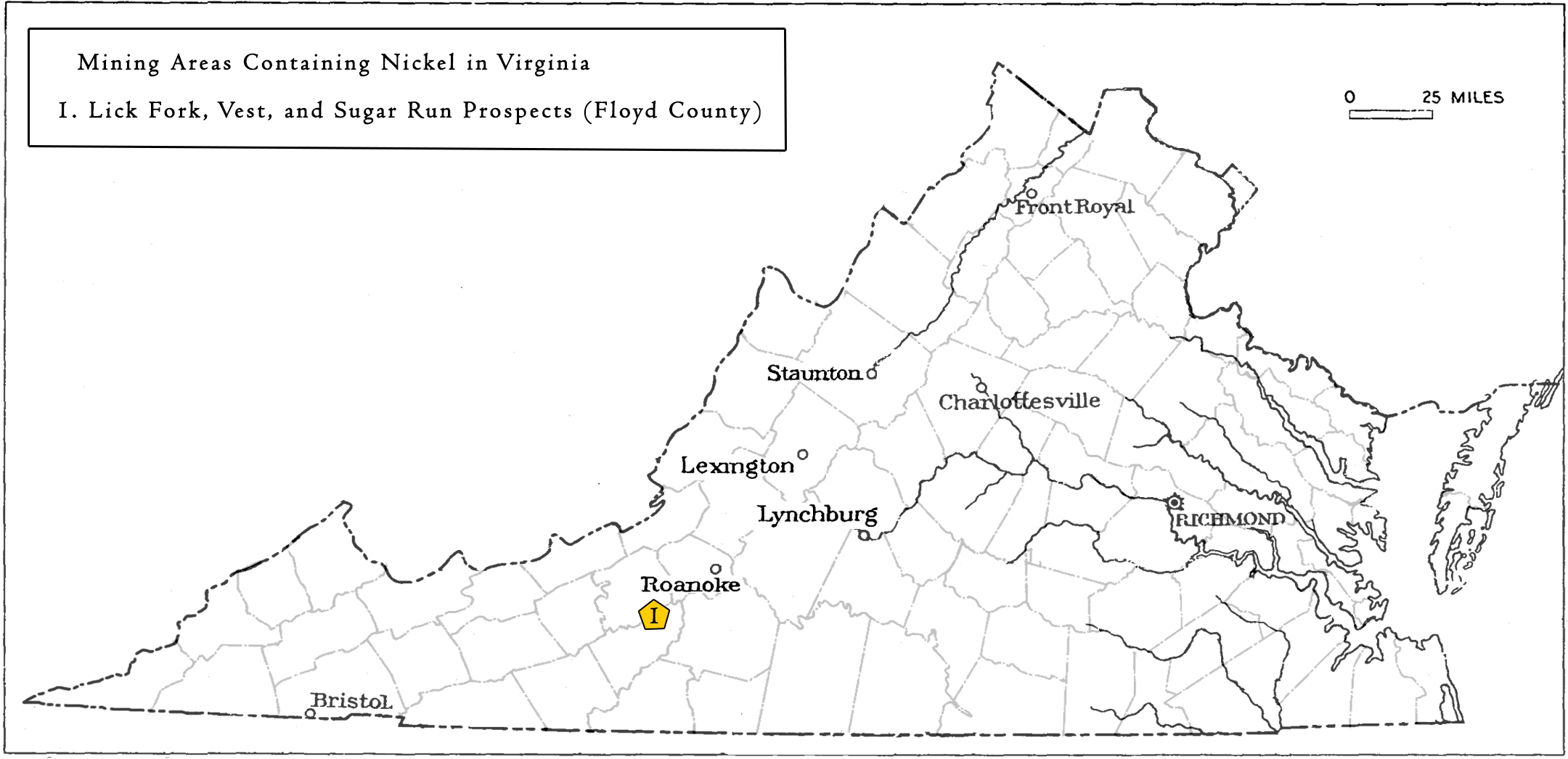
Locations in Virginia mined for Nickel
Dump from Vest Prospect next to a stream. Modified from Sweet and Bell, 1980.
Occurrences of iron, nickel, copper, and cobalt-enriched rocks are located in northern Floyd County (Watson, 1907). Three prospects were explored in the early 20th century with nickel as the primary commodity. Some of these locations were prospected intermittently for decades although none produced nickel on a commercial scale. At each of these prospects, iron-nickel-cobalt sulfides and iron titanium oxides occur in ultramafic or altered ultramafic bodies.
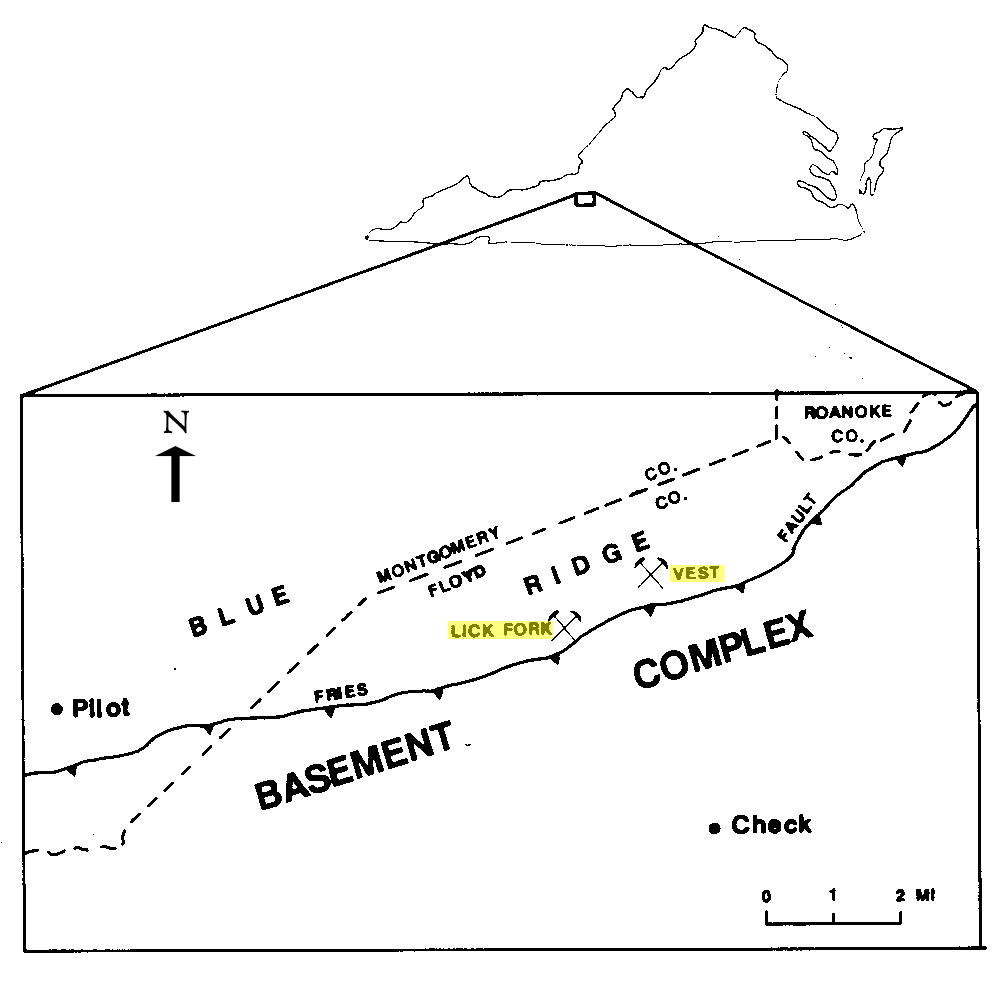
Location of the Lick Fork and Vest prospects, Floyd County, Virginia. Modified from Walsh-Stovall and others, 1989
The Lick Fork prospect (also known as Mackusick, Flat Run, and John Light's Mine) was operated in 1904, although sulfide-related ore had been identified in this location prior to the Civil War (Ross, 1935). Here, the sulfide ores are hosted by mafic and ultramafic rocks ranging in composition from pyroxene syenite to gabbro (Craig and Higgins, 1975). The ore bearing gabbro was estimated by Watson (1907) to be about 18-22 feet wide in the main adit. These rocks intrude medium-grained granulite-facies metamorphic rocks on the north side of the Fries Fault zone. The Lick Fork prospect was expanded from 1904 to 1907 by the Virginia Nickel Corporation, which opened several exploratory shafts and adits exposing disseminated and semi-massive sulfide zones. The minerals pyrrhotite, pentlandite, pyrite, chalcopyrite, ilmenite, magnetite, and violarite (a secondary oxidation mineral of pentlandite) were identified. Samples of violarite are reported to contain up to 30.7 weight percent nickel (Craig and Higgins, 1975). Geochemical surveys were performed at the Lick Fork and Vest prospects. The soil-sample analyses from both prospects show distinct nickel anomalies with values ranging up to 1000 ppm at the Lick Fork prospect (Walsh-Stovall and others, 1989).
At the Vest prospect, located about two miles to the northeast of Lick Fork, similar mafic intrusive rocks have undergone metamorphism resulting in chlorite-tremolite schist within biotite gneiss (Walsh-Stovall and others, 1989). The mafic intrusive rocks originally hosted a suite of sulfide minerals, now well oxidized, that are similar to that found at Lick Fork. Exploratory work in 1924 included a 15-foot vertical shaft and a 75-foot-long adit (Sweet and Bell, 1980; Grosh, 1949). Four exploratory holes were drilled in 1936, and two more were added by the U.S. Bureau of Mines in 1944 (Walsh-Stovall and others, 1989). Samples reveal sparsely disseminated sulfide zones measuring up to 5 feet in thickness and containing 3.2 percent nickel (Grosh, 1949). The soil-sample analyses show up to 425 ppm at the Vest prospect (Walsh-Stovall and others, 1989). There are no records of mineral production from either Lick Fork or Vest (Sweet and Bell, 1980; Sweet and others, 2016).
A third nickel prospect, named Sugar Run, was explored in 1902. Approximately three miles northeast of the Vest prospect, Sugar Run consisted of small underground workings, including one shaft and a 40-foot adit (Dietrich, 1959). This location did not produce commercial quantities of ore.
Selected References:
Craig, J.R., and Higgins, J.B., 1975, Cobalt- and Iron-Rich Violarites from Virginia. American Mineralogist, Volume 60.
Dietrich, R.V., 1959, Geology and Mineral Resources of Floyd County of the Blue Ridge Upland, Southwestern Virginia. Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series, No. 134.
Grosh, W.A., 1949, Investigation of Vest nickel prospect, Floyd County, Virginia: U.S. Bureau of Mines Report of Investigations R.I. 4491, 4 p.
Hofstra, A.H., and Kreiner, D.C., 2020, Systems-Deposits-Commodities-Critical Minerals Table for the Earth Mapping Resources Initiative: U.S. Geological Survey Open-File Report 2020-1042.
Ross, C.S., 1935, Origin of the Copper Deposits of the Ducktown type in the Southern Appalachian Region, U.S. Geological Survey, Professional Paper 179.
Sweet, P., and Bell, S., 1980, Metallic mineralization in the Blue Ridge province of Virginia, in Virginia Division of Mineral Resources Publication 27, p39-53.
Sweet, P.C., Lassetter, W.L., and Sherwood, W.C., 2016, Non-Fuel Mineral Resources in Virginia, in The Geology of Virginia. Virginia Museum of Natural History, Special Publication 18.
Walsh-Stovall, C., Robinson, E.S, Rimstidt, J.D., and Stovall, R.L., 1989, Exploration for Magmatic Sulfide Deposits in the Virginia Blue Ridge, in Contributions to Virginia Geology - VI, Virginia Division of Mineral Resources, Publication 88.
Watson, T.L., 1907, Mineral Resources of Virginia, Lynchburg, VA, J.P. Bell Company, 618 p.
.jpg)
