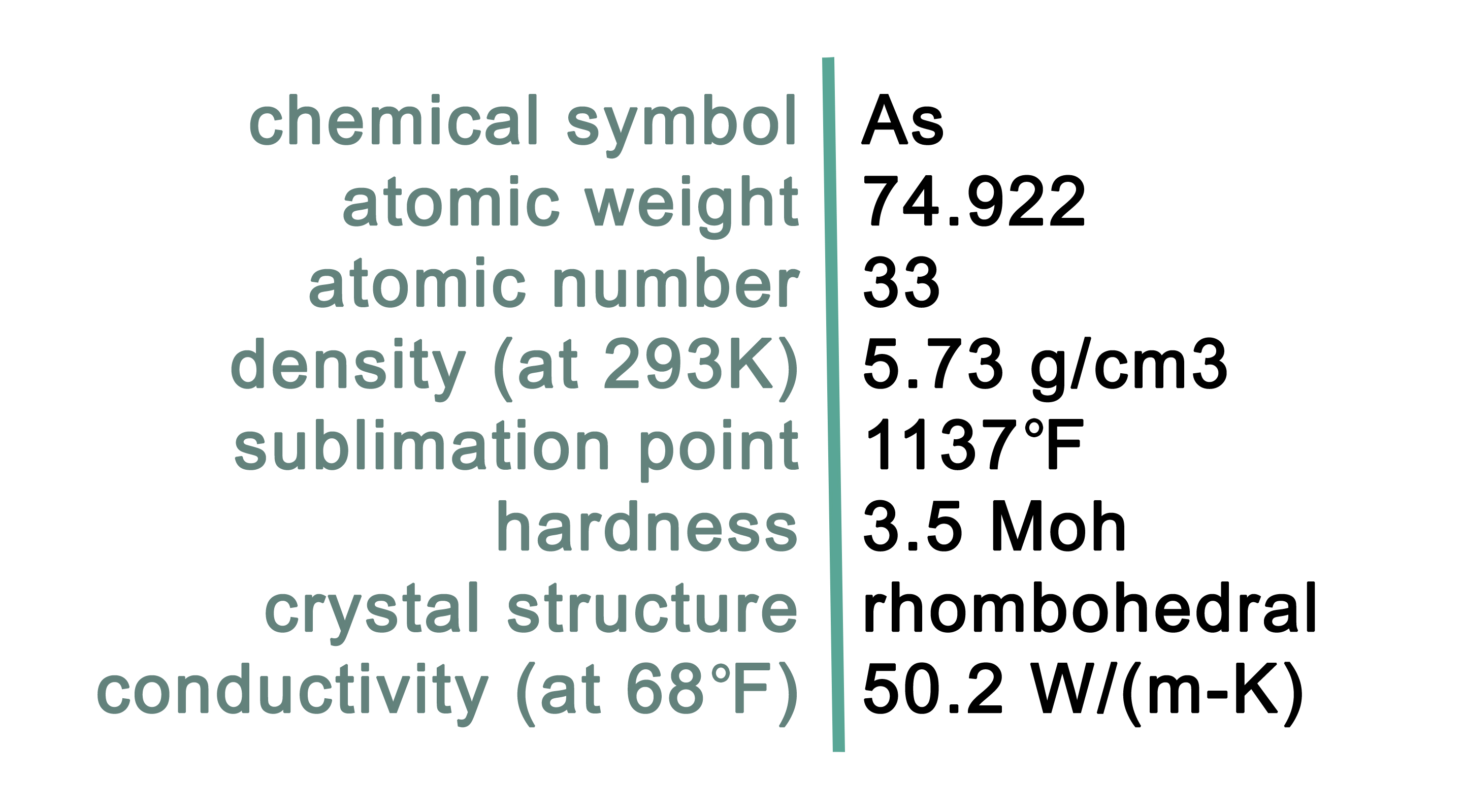
Characteristics of Arsenic
Arsenic is a gray metal with the chemical symbol As. As a pure metal, arsenic is semi-metallic and very brittle. Arsenic reacts rapidly with air, tarnishing and oxidizing into arsenous oxide. When heated, arsenic sublimes forming ferrous sulfide. It is a strengthening alloy with other metals, although tends to be soft in pure form. Arsenic does not melt, but sublimes directly into gas at high temperatures. It is a poor conductor. Arsenic is poisonous. Arsenic is used predominantly as a pesticide treatment for wood. Pure arsenic is rare on Earth, although arsenic is commonly bound with other elements as various minerals found across the globe. Arsenic is commonly in the minerals listed in table 1.

| Mineral Name | Chemical Formula | Specific Gravity | As % |
|---|---|---|---|
| Arsenopyrite | FeAsS | 6.19 gm/cc | 46.01 |
| Safflorite | (Co,Fe)As2 | 7.48 gm/cc | 72.04 |
| Glaucodot | (Co,Fe)AsS | 6.22 gm/cc | 45.37 |
| Skutterudite | CoAs3 | 5.95 gm/cc | 76.09 |
| Realgar | AsS | 3.60 gm/cc | 70.03 |
| Orpiment | As2S3 | 3.49 gm/cc | 60.90 |
Table 1: Minerals containing the element Arsenic

Uses of Arsenic
Arsenic was used in ancient times as a poison, as an alloy to strengthen bronze, and even as a topical to lighten skin. Although widely used in historic times, health and environmental concerns have led to more limited modern use. Today, arsenic is considered a "critical mineral" in domestic metallurgical applications that serve defense, energy, and telecommunications technologies (Fortier and others, 2018). It is primarily used as a pesticide for preserving lumber (pressure treated wood) and for various agricultural chemicals. Arsenic compounds are used to make components in semi-conductors for electronics, photovoltaics, telecommunications, aerospace research, and biomedical applications. This element is also used as an alloy to harden lead in car batteries, and as an alloy with other metals to reduce corrosion and improve strength.

A realgar crystal.
Photo credit: Robert Lavinsky
Arsenic Geology
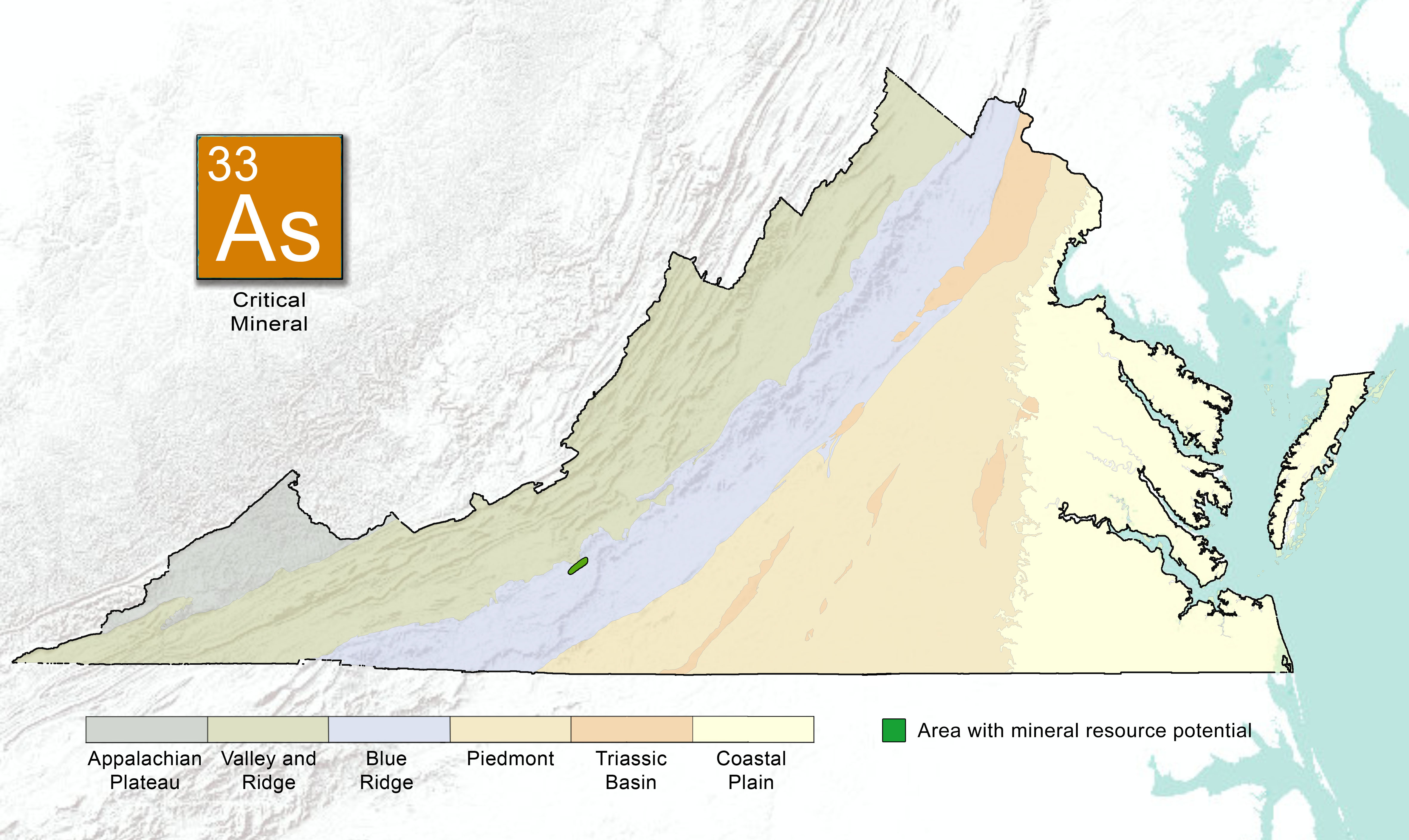
Arsenic is common in the Earth's crust, bound with other elements to form a variety of minerals. Arsenic-bearing minerals may form within igneous intrusive or metamorphic rocks. Hydrothermal environments including hot springs, certain volcanic environments, and areas of contact metamorphism may host arsenic-bearing minerals. Arsenic may also form in igneous pegmatite rocks. Arsenic is often associated with metallic or sulfide deposits such as gold and copper ores.
| Mineral System | Deposit Type | Geologic Provinces |
|---|---|---|
| Arsenide | Post-magmatic arsenopyrite in greisen | Blue Ridge |
Table 2: Prospective arsenic mineral systems, deposit types (Hofstra and Kreiner, 2020), and geologic provinces in Virginia
Arsenic in Industry
Arsenic is often obtained as a smelting byproduct of copper, gold, iron, and lead. Arsenic is largely mined in China and Morocco (USGS, 2020). The U.S. does not maintain a supply of arsenic in the National Defense Stockpile and is 100 percent import reliant from several countries including China, Morocco, Japan, and Belgium. Arsenic has not been produced in the United States since 1985. Concerns related to the mining and production of arsenic include release of arsenic into soil and water where contamination is a serious health issue.
In Virginia, although arsenic may exist within many sulfide deposits, only one arsenic mine has produced historically. The arsenic discovered and mined in the Commonwealth is associated with other sulfide or metallic minerals and elements such as pyrite, gold, copper, iron, cobalt, and nickel. These sulfide ores can be found in veins, or irregularly distributed throughout the hosted metamorphosed volcanic or magmatic rocks.
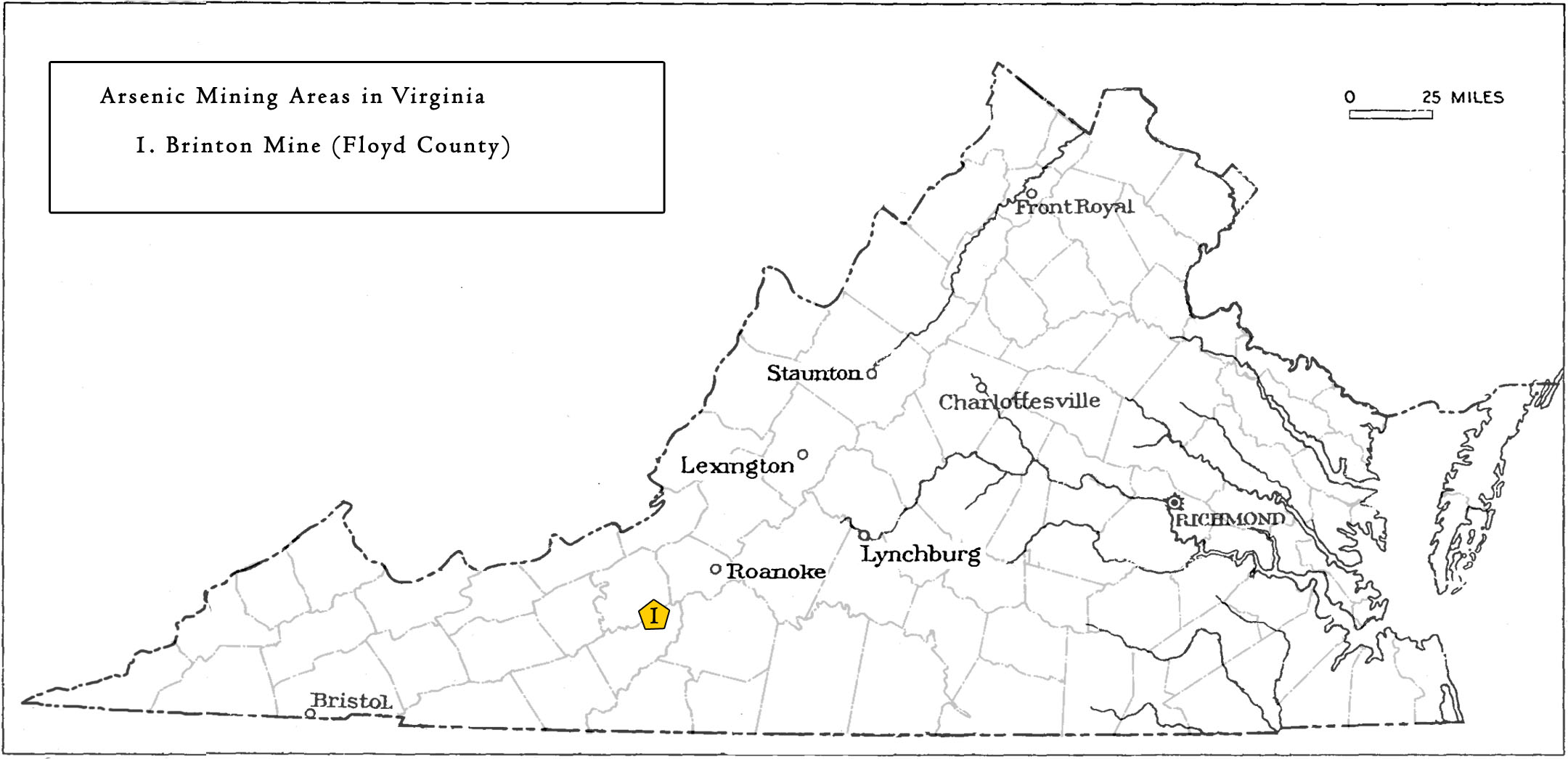
Locations in Virginia mined or prospected for Arsenic
Arsenic was discovered in Floyd County as early as 1882 (Hess and Graton, 1911). Production did not occur until the construction of the Brinton Mine near Huffville in 1902 (Hess and Graton, 1911; Luttrell, 1966). Primarily within the Lynchburg gneiss, the arsenic is found as arsenopyrite intergrown with pyrite occurring in lenticular veins and disseminations. The ore body is 3 to 14 feet thick and reaches a depth of 120 feet. In 1902, a processing plant was built at the mine site and between the years 1903 and 1917, the mine produced up to 100 tons of arsenic (Luttrell, 1966; Young, 1956). The arsenic produced here was used to manufacture insecticides (Hess and Graton, 1911). The processing mill was dismantled in 1919 and mining has not recurred since (Luttrell, 1966).
Selected References:
Cowan, J.L., 1904, The arsenic mines at Brinton, Virginia: Engineering and Mining Journal, v. 78, p. 105-106.
Dietrich, R.V., 1959, Geology and Mineral Resources of Floyd County of the Blue Ridge Upland, Southwestern Virginia. Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series, No. 134.
Dietrich, R.V., 1959, Geology and Mineral Resources of Floyd County of the Blue Ridge Upland, Southwestern Virginia. Bulletin of the Virginia Polytechnic Institute Engineering Experiment Station Series, No. 134.
Fortier, S.M., Nassar, N.T., Lederer, G.W., Brainard, J., Gambogi, J., and McCullough, E.A., 2018, Draft Critical Mineral List - Summary of Methodology and Background Information - U.S. Geological Survey Technical Input Document in Response to Secretarial Order No. 3359: U.S. Geological Survey Open-File Report 2018-1021, 15 p.
Hess, H.H., and Graton, L.C., 1911, The arsenic deposits of Brinton, Virginia: U.S. Geological Survey Bulletin 470E, p. 205-211.
Hofstra, A.H., and Kreiner, D.C., 2020, Systems-Deposits-Commodities-Critical Minerals Table for the Earth Mapping Resources Initiative: U.S. Geological Survey Open-File Report 2020-1042.
Luttrell, G.W., 1966, Base- and Precious-metal and Related Ore Deposits of Virginia. Virginia Division of Mineral Resources, Mineral Resources Report 7.
Magee, M., 1968, Geology and Ore Deposits of the Ducktown District, Tennessee, in Ridge, J.D. [ed], Ore Deposits of the United States, 1933-1967: American Institute of Mining, Metallurgical, and Petroleum Engineers, Graton-Sales volume I.
Ross, C.S., 1935, Origin of the Copper Deposits of the Ducktown type in the Southern Appalachian Region, U.S. Geological Survey, Professional Paper 179.
Sweet, P.C., Good, R.S., Lovett, J.A., Campbell, E.V.M., Wilkes, G.P., and Meyers, L.L., 1989, Copper, lead, and zinc resources in Virginia: Virginia Division of Mineral Resources Publication 93, 185 p.
Sweet, P.C., Lassetter, W.L., and Sherwood, W.C., 2016, Non-Fuel Mineral Resources in Virginia, in The Geology of Virginia. Virginia Museum of Natural History, Special Publication 18.
Walsh-Stovall, C., Robinson, E.S, Rimstidt, J.D., and Stovall, R.L., 1989, Exploration for Magmatic Sulfide Deposits in the Virginia Blue Ridge, in Contributions to Virginia Geology - VI, Virginia Division of Mineral Resources, Publication 88.
Watson, T.L., 1907, Mineral Resources of Virginia, Lynchburg, VA, J.P. Bell Company, 618 p.
Young, R.S., 1956, Sulfides in Virginia: Division of Mineral Resources Virginia Minerals v. 2, No. 1, p. 1-7.
Young, R.S., 1981, Sulfide zones, in Geologic investigations in the Willis Mountain and Anderson quadrangles, Virginia: Virginia Division of Mineral Resources Publication 29, p. 17-47.

.jpg)
